 |
| Source: https://goo.gl/vQx81a |
Summary:
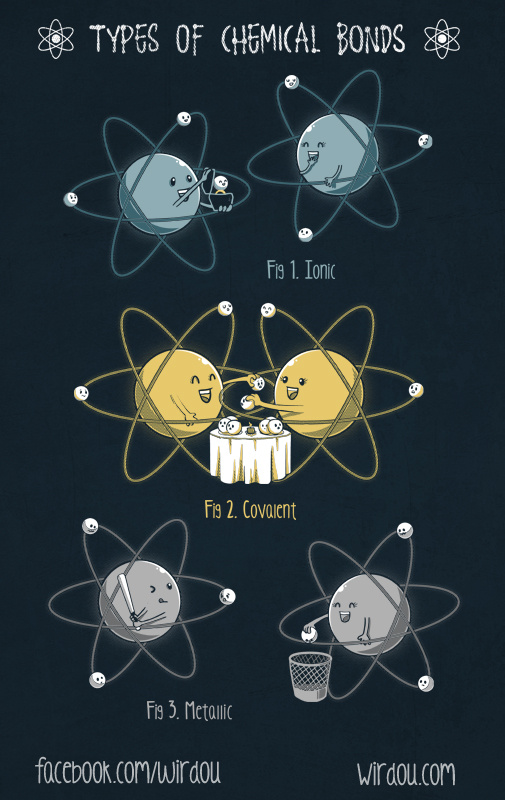
When one or molecules mix together as reactants, a chemical reaction sometimes occurs, making a product. There are some rules that happen in every chemical reaction. The Octet Rule states that atoms will gain or lose electrons in order to obtain 8 electrons in its outer shell so it. This is so it can act similar to a noble gas. The Law of Conservation of Mass states that atoms cannot be destroyed nor created during a chemical reaction. The product is simply a rearrangement of the reactants and the chemical equations are balanced. When a chemical reaction occurs, there are bonds that happen. A covalent bond is when atoms share electrons in order to gain 8 electrons in its outer shell. An ionic bond occurs when one atom takes an electron from another atom, making one atom a positive ion and another a negative ion, which means that they attract each other. Ionic bonds can create ionic crystals which means that an ionic bond attracts similar ionic bond pairs until there are no more nearby.
SP5 - Using Mathematics:
This week I used mathematics to count how many atoms were in a formula. I used the subscript next to an element to find how many of that element was in the formula. If the subscript was to the right of elements enclosed in parenthesis, that meant that you had to multiply the subscript by how many of each element you had in the parenthesis. For example, if I had (H2 + O)2, there would be 4 hydrogen atoms and 2 oxygen atoms since you distribute the 2 among both elements in the parenthesis. In addition to having a subscript, there was a co-efficient which showed how much of each element there was to the right of that subscript. The equation 2H + Cl meant that there were 2 hydrogen atoms and 2 Chlorine atoms since you are supposed to multiply 2 by anything to the right of it. If there was a parenthesis with a subscript and a co-efficient, you would first remove the parenthesis is then remove the co-efficient
XCC - Scale, Proportion and Quantity:
Something that is affected by scale is ionic bonds and chemical reactions. When an ionic bond forms, it can attract other similar pairs of ionic bonds in order to create ionic crystals, such as salt. It is affected by scale due to the fact that when the bond is created, it can attract other bonds to become bigger. Chemical reactions are also something that are affected by scale. Take the reaction of vinegar and baking soda. When you mix vinegar and baking soda together, it foams and expands, also creating carbon dioxide. If you add more vinegar and/or baking soda, it can create more carbon dioxide and expand more and is thus affected by scale.
No comments:
Post a Comment